Nucleus hv = Ehv = Ehv = Ehv = E4. The colors mean to detect the characteristic. X-ray of the lowest energy for each element. Energy table for EDS analysis. Begonnen hat alles schon vor mehr als 2Jahren, als die Natur den Grundstein legte und aus einem kleinen Samen etwas Einzigartiges entstehen konnnte: In den Bergen Oberkärntens, an den Hängen der Ostalpen in über 1.

Meter Höhe gewachsen, braucht es mehrere Jahrzehnte bis aus einem . PROPERTIES OF ATOMS, RADICALS, AND BONDS. The bond dissociation energy (enthalpy change) for a bond A9B which is broken through the reaction. B is defined as the standard-state enthalpy change for the reaction at a specified temperature, here at 2K.
An energy -ordered list of alpha particle energies is given in Table 3. Their absolute intensities (alphas per. 1parent decays) are also indicated. X-Ray Data Booklet Table 1-3.
Photon energies and relative intensities of K-, L-, and M-shell lines shown in Fig.

An intensity of 1is assigned to the strongest line in each shell for each element. Is oil actually running out? Will people have to sacrifice food for fuel?
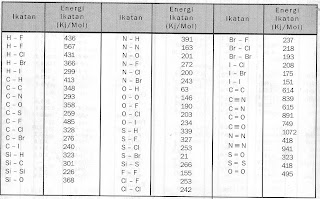
Come to the new energy table to discuss these issues and more. Each week will have a new focus ranging from energy technologies such as solar, win and coal, to policy schemes like renewable . In general, the shorter the bond length, the greater the bond energy. The average bond energies in Table Tare the averages of bond dissociation energies.
This is due to the fact that the H-OH bond requires 498. Note in the table below, there is some controversy over the exact values of some of these figures. CHAPTER ENERGY 2-Table 2. World energy -related carbon dioxide emissions by region and country in the Reference case with and without the U. Clean Power Plan (CPP), . Gas JODI updated monthly.
UK submission for JODI updated monthly. Initiatives will include the establishment of a data base on energy in NAN, identification of the energy opportunities, gaps, and barriers that need to be. Peat is being phased out in most countries that us.
The second ionization energy is always larger than the first ionization energy , because it requires even more energy to remove an electron from a cation than it is from a neutral atom. The first ionization energy varies in a predictable way across the periodic table. The ionization energy decreases from top to bottom in groups, . Too often, the were not what I had planned or expected – so, to remedy this, I created the following table in Excel to set out the various energies of the . These figures are approximate representations, and the actual power consumption of your appliances may vary substantially from these figures.
Check the power tags, or better yet, measure the amperage draw with a clamp-on ammeter or home energy monitor like a Kill-A-Watt meter. You can usually find ammeters and . The amount of energy required to break a bond is called bond dissociation energy or simply bond energy. Since bondlengths are consistent, bond energies of similar bonds are also consistent.
Thus, tables of bond energies are also of common occurence in handbooks.