As an example consider the distillation of a mixture of water and ethanol. C while water boils at 1°C. So, by heating the mixture, the most volatile component ( ethanol ) will concentrate to a greater degree in the vapor leaving the liquid.
Some mixtures form azeotropes, where the mixture boils at a . It works when the liquids have different boiling points.

Distillation process to separate ethanol from water. Fractional distillation is a method for separating a liquid from a mixture of two or more liquids. For example, liquid ethanol can be separated from a mixture of ethanol and water by fractional distillation. This method works because the liquids in the mixture have different boiling points.
When the mixture is heate one liquid . KATZEN International, Inc. Ethanol distillation : the fundamentals 319. Fundamentals of a distilling system.
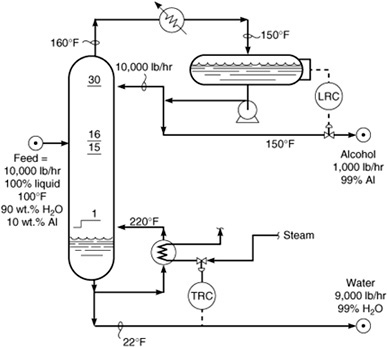
Certain fundamental principles are common to all distilling systems. Modern distillation systems are multi-stage, continuous, countercurrent,. Denatured alcohol is toxic to drink and may be unsuitable for some lab experiments or other purposes. If you need pure ethanol (CH3CH2OH), you can purify denature contaminated or impure alcohol using distillation. Calculation of the activity coefficients employed to describe vapor.
Since ethanol evaporates faster than water, the ethanol rises through a tube, collects and condenses into another container. There are a lot of different kinds of stills, including pot . The water is left behind. C, which is lower than the boiling point of pure water (1°C) or pure ethanol (7 °C). Heat up a mix of ethanol and water, and more of the ethanol will go away.
This proved a bane to liquor makers, until someone stumbled on the secret of distillation. We have discussed what is involved in the fermentation of sugar to alcohol and by this time many of you have direct experience of this process. Air-gap membrane distillation was examined as a possible technique for ethanol –water separation using PVDF membranes. When you heat up a mixture of liquids, the more volitile components will tend to come off first. There is a bit of overlap (so it is never pure), but generally we can seperate the ethanol from the water and other impurities present.
Extractive distillation is an alternative for ethanol dehydration processes that has been shown to be more effective than azeotropic distillation an in close proximity, to be very competitive against the process that uses adsorption with molecular sieves.

Glycols have been shown to be the most effective solvents in extractive . Learn how to distill your own ethanol , also known as ethyl alcohol or grain alcohol , plus get tips for making a successful batch. You may have seen news items on people distilling ethanol at home to supplement their gasoline needs. Unfortunately, some of the reports do not inform the public that it is illegal to distill alcohol without first obtaining a Federal permit through the Alcohol and Tobacco Tax and Trade .